Stem Cell Research Sheds Light on Age-Related Osteoporosis
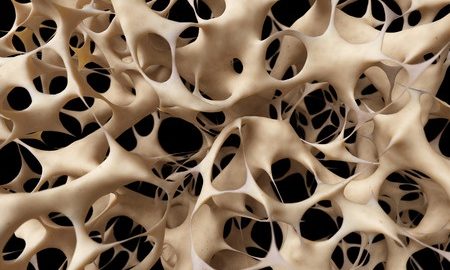
Researchers at China’s Zhejiang University and the University of Alabama in Birmingham have mapped cell mechanisms and shed light on the development of age-related osteoporosis.
Age-Related Osteoporosis: An Overview
Osteoporosis is known as the brittle bone disease. The condition is associated with weak bones that are at an increased risk of fractures. In healthy people, there is a balance between the formation of new bone and the removal of old bone. This balance ensures that healthy bone constantly replaces old bone. With advancing age, the rate at which old and damaged bone is lost is more than the rate of new bone formation. This leads to an overall reduction in bone density. Such a condition when bone is more porous is known as osteoporosis.
It is estimated that more than 50 million Americans have osteoporosis. One in two people over the age of 50 have low bone mass. Approximately 50 percent of women and 25 percent of men over the age of 50 suffer a broken bone due to osteoporosis. Fractures are the most serious complication of the disease and are associated with high morbidity and mortality, especially in older patients. Researchers have been trying to find new drugs and treatments for osteoporosis which is a silent disease that can sneak up on patients.
Progenitor Cells and Bone Biology
Immature cells that have the ability to differentiate into different types of cells are called progenitor cells. Genetic instructions and transcription factors determine the type of tissue these cells transform into. Mesenchymal stem cells in the bone marrow are the progenitor cells for bone tissue.
New Research Sheds Light on Osteoporosis
By studying the cell mechanisms involved in bone loss in aging individuals, the investigators hope to better understand the biology of osteoporosis and develop new treatments for the disease. The researchers in China and Alabama have identified a protein called Cbf-beta that is critical in controlling the maturation of progenitor cells into bone cells and therefore the rate of new bone formation.
The core binding factor (Cbf) is a transcription factor that is vital in maintaining the balance between bone loss and bone creation. The researchers deleted Cbf-beta in laboratory mice to disrupt the maturation of progenitor cells. This was done in three populations of mice at three different stages of the process. This led to osteoporosis in all three populations with fat cells replacing bone cells, a pattern that is similar to humans with age-related osteoporosis. Further investigations revealed that Cbf-beta prevents progenitor cells from maturing into fat cells by activating a cell signal.
It is hoped that the insight gained from the cell mapping and the importance of Cbf-beta will help develop novel treatments for bone loss with minimal adverse effects.
References:
- https://www.medicalnewstoday.com/articles/319543.php
- https://www.nof.org/patients/what-is-osteoporosis/


