Stem Cell Therapy for Duchenne Muscular Dystrophe
A study sponsored by Stem Cells Arabia is investigating the safety and efficacy of autologous marrow-derived stem cell therapy in children and adults with Duchenne muscular dystrophy.
Duchenne Muscular Dystrophy
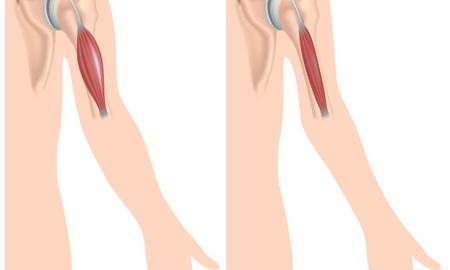
Duchenne muscular dystrophy (DMD) is an X-linked genetic disorder that primarily affects boys and is characterized by progressive muscle weakness. The condition is caused by the genetic absence of dystrophin protein which is essential for the integrity of muscle cells. Symptoms typically manifest at a young age (as early as 3-5 years old) and usually begin with a weakness of the pelvic and lower limb muscles, spreading progressively to the upper extremities and trunk. By the time the affected person is in their teens, the muscles of respiration and the heart are also affected.
There is no cure for DMD. Current treatment is symptomatic, consisting of braces, physical therapy, and corrective surgery. Pharmacologic treatment can include steroids, anticonvulsants, and immunosuppressants to control symptoms. Advanced medical care allows boys with DMD to live into their early 30s and there are reports of survival into the 40s and 50s.
Stem Cell Therapy for Duchenne Muscular Dystrophy
There are several exciting treatment strategies involving gene therapy and gene repair that are being investigated to find a cure for Duchenne muscular dystrophy. Stem-cell-based therapies are a promising avenue of treatment for this debilitating condition. There are two key approaches to cell therapies for DMD. One is to genetically alter cells from a patient with DMD so that dystrophin expression is restored. These repaired cells are then re-implanted into the patient through an autologous stem cell transplant. An allogeneic stem cell transplant, on the other hand, involves a transfer of functional dystrophin-producing cells from a healthy individual.
New Study for Duchenne Muscular Dystrophy
Researchers have designed a single-arm single-center clinical trial to test the safety and efficacy of mesenchymal stem cell treatment for DMD. Marrow-derived autologous mesenchymal stem cells will be administered in this open-label single group interventional study and outcomes will be measured in terms of improved muscle strength measured by MMT or kinetic muscle testing 12 months after the infusion. The Brooke and Vignos Scale will also be measured. The study expects to enroll 20 patients aged between 4 and 25 years old. Healthy people are not being accepted into the study as volunteers. The results are expected in March 2020.
References:
- https://clinicaltrials.gov/ct2/show/NCT03067831?term=stem+cells&sfpd_s=01%2F01%2F2017&sfpd_e=10%2F19%2F2017&draw=1&rank=4
- https://www.mda.org/disease/duchenne-muscular-dystrophy


